Many recent articles have raised the idea that gas stoves are harmful to health:
the concentration of harmful nitrogen oxides – a byproduct of burning natural gas – can reach or surpass a healthy limit within minutes, especially in homes with small kitchens.
the evidence against gas stoves is damning. […] Homes with gas stoves can contain approximately 50 to 400 percent higher concentrations of NO2 than homes with electric stoves
compelling evidence that gas appliances can produce levels of air pollution inside homes that would be illegal outdoors in the United States
Why Gas Stoves Are More Hazardous Than We’ve Been Led to Believe (Slate)
Let’s agree that gas stoves affect health, but how much? After all, someone who loves gas cooking might rationally view some level of harm as an acceptable tradeoff.
Now, gas stoves appear to increase the odds of asthma in children, perhaps by around 40%. This is important, but I wanted to know something else: What do gas stoves do to life expectancy? I couldn’t find any concrete estimates.
So I made one. My best guess is that if you use a gas stove for your entire life instead of an electric stove and change nothing else, that shortens your life expectancy by around 53 days on average. That’s assuming you have a well-maintained stove with an electronic ignition. If you have a pilot light instead, you should increase it to 88 days. If the stove is broken, it could be worse again.
Overview
How do gas stoves hurt you?
They create various kinds of indoor air pollution. The most important difference from electric stoves is that they create more nitrogen dioxide (NO2). This hurts you because it has an extra unpaired electron so that after it gets into your respiratory system it reacts with many random molecules, causing problems like inflammation.
How much does NO2 hurt you?
If someone is exposed to an additional 1 μg/m3 of NO2 for their entire life our best estimate is that decreases their life expectancy by around 7.3 days. This number comes from looking at mortality statistics for large groups of people and relating them to outdoor NO2 levels.
How much does having a gas stove increase NO2 over an electric stove?
On average, by around 7.25 μg / m3 for a gas stove without a pilot light, and by around 12.0 for a gas stove with a pilot light. This is estimated by measuring levels in different types of homes, or by actually switching stoves out and seeing what happens.
What’s the math?
A gas stove without a pilot light costs 7.25 × 7.3 days = 52.9 days. A gas stove with a pilot light costs 12.0 × 7.3 days = 87.6 days.
Can a range hood fix this?
Yes, if you have a sufficiently powerful hood with a fan that vents outside. When Singer et al. checked this in 9 homes with externally venting hoods, they got a reduction of around 90% in one home, 70% in a second, 50% in a third, and 30% or less in the other six.
Can an air purifier fix this?
A HEPA filter does nothing. Activated charcoal could work in principle, but the (minimal) experimental evidence we have isn’t encouraging.
How uncertain is this estimate?
It’s probably correct within an order of magnitude or so on average. In addition, the amount of NO2 your stove puts out or the harms of NO2 to your health may vary from the average.
Why might this be wrong?
I’m not accounting for particle pollution since that mostly comes from the food and can be solved with an air purifier. I’m also not accounting for direct methane emissions (gas stoves leak a bit all the time.) or other products of burning methane which I think are less important.
Also, the estimate of how much NO2 hurts you might be off. This estimate uses observational data of outdoor levels to try to infer a causal influence. That’s always hard, and outdoor levels are much less spiky than indoor levels, which could be important if the body’s response to NO2 is nonlinear.
Isn’t 53 days tiny and ignorable?
I wouldn’t ignore it. As they say, “53 days here, 53 days there, pretty soon you’re talking about real money.” People choose painful and expensive medical procedures with smaller benefits all the time. And remember, it’s 53 days on average: many people lose less and some people much more.
But if you’re really sure you don’t care about 53 days, well, in theory it might be exciting to learn that a possibly exciting thing isn’t exciting after all?
I emphasize that this is my best guess, which I’m publishing this on the theory that an imperfect answer to an important question is valuable. But I don’t mean to hide behind that excuse so, to assist you in pointing out that I’m wrong and terrible and bad, I’ve added a superscript of ᵈ to statements that I particularly doubt.
What happens when you burn natural gas?
Natural gas is methane, CH4 (with a tiny amount of methanethiol added to make it smell). When it’s burned, the intended reaction is
(1.48 × 10-18 J of heat, to be precise.) You can also get incomplete combustions like
which results in soot or
which produces carbon monoxide. However, in a properly functioning gas stove very little incomplete combustion will happenᵈ.
However, the major way that gas stoves affect health seems to be a different chemical entirelyᵈ.
What is NO2?
It’s a molecule that looks like this:
The half dashed connections indicate partial double bonds, halfway between a single bond and a double bond. These leave an extra unpaired electron, meaning NO2 is a free radical.
Where does NO2 come from?
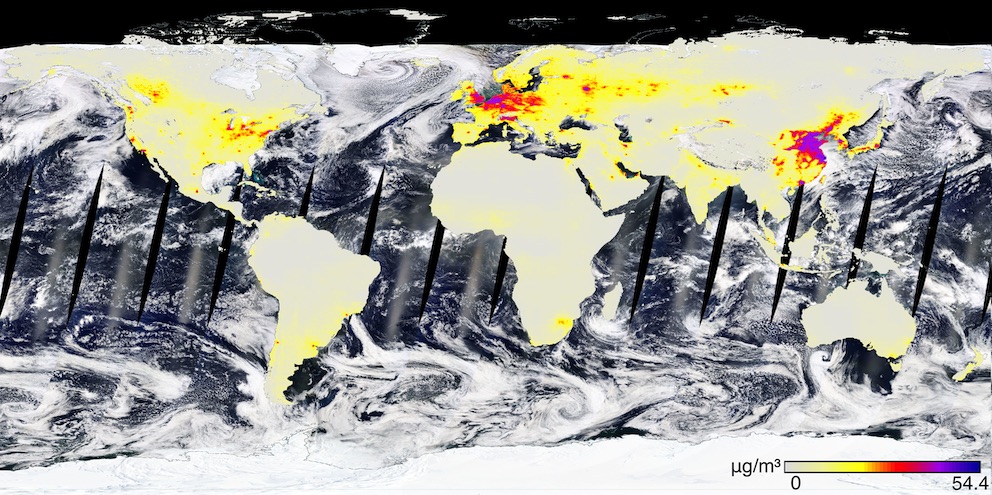
Air on Earth is mostly nitrogen gas (N2). At room temperatures nitrogen is inactive but at high temperatures, the two nitrogen atoms can split apart and then react with oxygen to form nitrogen oxides, e.g. nitric oxide (NO) or nitrogen dioxide (NO2). The largest natural sources of NO2 are forest fires and—amazingly—lightning strikes. (I was only able to accept this after learning that the Earth has around 100 lightning strikes per second.) This leads to natural background concentrations that would average between 0.4 and 9.4 μg/m3 in different parts of the planet if there weren’t humans scurrying around everywhere creating more.
Speaking of humans: We love burning stuff, so NO2 levels tend to be higher in cities. Here are the average outdoor levels between 2010 and 2012, courtesy of NASA. The major change since an earlier 1996-1998 survey is that levels decreased a lot in North America, decreased moderately in Europe, and increased a lot in China.
(Click to see a higher resolution version.)
NO2 doesn’t hang around in the air for that long. Within 5-10 days it tends to convert to nitric acid (HNO3) and rain out. This makes the impact of these emissions fairly local. If you look closely at the above map, for example, you’ll see that even though Northern Europe looks very clean, there are small red dots over almost all the population centers.
But what does all this have to do with natural gas? Well, burning methane is just like burning anything else: it produces heat, and that heat makes NO2.
How does NO2 hurt you?
Theory
Free radicals like NO2 are highly reactive and intrude into all sorts of biological reactions in cells. This produces unexpected chemicals and inflammation, wherein your immune system goes on a little rampage. Free radicals can also react with the chemicals that make up our DNA, leading to mutations and potentially cancer. This whole situation with free radicals is called oxidative stress. Some amount of oxidative stress is normal since cells produce free radicals as part of standard operations all the time. As a defense mechanism, the body makes anti-oxidant chemicals that aggressively bind with free radicals and neutralize them. But if too many free radicals show up at once these defenses could be overwhelmed and damage could quickly stack up.
Experiments
That’s the theory. What do the experiments say? Here’s my best attempt to summarize the WHO’s summary of the evidence.
First, there are lots of experiments on human cell lines, usually taken from somewhere in the respiratory tract. These are broadly consistent with the theory above regarding inflammation, though they almost always use extremely high concentrations (e.g. 85,000 μg/m3 of NO2). A common experiment would be to stress some cells to try to mimic a condition like asthma and then expose them (or not) to NO2. Usually, they find that the cells die more easily with the NO2, though sometimes there’s little or no effect is observed at all. There was one experiment with an exposure as low as 200 μg/m3 that did see some inflammation.
Second, there are also experiments on animals. Typically they find that if they stress the animals and then expose them to these high levels of NO2 it enhances the damage of the stress. But this is highly dependent on the animal and these experiments almost always used levels in the thousands or above. Many experiments found little or no effect.
Third, there are controlled exposures in humans. These are always short-term, presumably because people don’t like being confined to a room with a possibly harmful gas for months at a time. They generally find that exposure to very high levels causes some kind of vaguely worrying change, like lung resistance or inflammation (or, more typically, something obscure like glutathione levels or responsiveness to methacholine, whatever that is).
There have been some experiments related to cancer, but they were relatively short term (e.g. 18000 μg/m3 for 6 hours) and weren’t able to find any impact on cancer or DNA damage.
Various experiments (both in cell lines and animals) compare different dosing regimes: For example, is it worse to have a continuous exposure of X μg/m3 or to have a 1-hour per day “peak” exposure of 24 X μg/m3. These usually find a stronger effect with the latter.
My basic conclusions from all this are:
- The theory regarding inflammation is probably true, but it’s only really confirmed at super-high doses (tens of thousands of μg/m3). Experiments even at high doses (thousands of μg/m3) may not find an effect.
- The story regarding cancer hasn’t really been confirmed at all, but we haven’t looked very hard.
- The dose-response curve might be nonlinear, meaning you lose information by just looking at the average “area under the curve” exposure.
- NO2 may be more harmful if have a respiratory condition.
How much does NO2 hurt you?
Wang et al. (2021) do a meta-analysis of the association between short-term exposure to NO2 and all-cause mortality, with a final estimate that an increase of 10 μg/m3 is associated with an increase in all-cause mortality of 0.87%. There are two problems with this. First, it just uses studies on short-term exposure, i.e. if levels go up in a city for a short time, how much does that increase mortality during that period. This will tend to underestimate the effects. Second, it doesn’t control for other pollutants, most notably for increases in particulate pollution. This will tend to (perhaps vastly) overrate the effects. I think this estimate is useless for our purposes.
Then, there are the long-term epidemiological studies. To give a flavor of these, I’ll describe one in detail. Fisher et al. (2015) took all the people in the Netherlands who in 2004 were at least 30 years old and who had lived at the same address since 1999. They then followed these people until 2011 and looked at if they died. They collected these data for each person:
- Age
- Sex
- Country/Region of origin
- Marital status
- Household income
- Socioeconomic status (estimated at the postal code level of around 4000 people each)
- Annual mean particle exposure (PM10) at their address
- Annual mean NO2 exposure at their address
Here is the model of the annual mean particulate (PM10) and NO2 pollution they used:
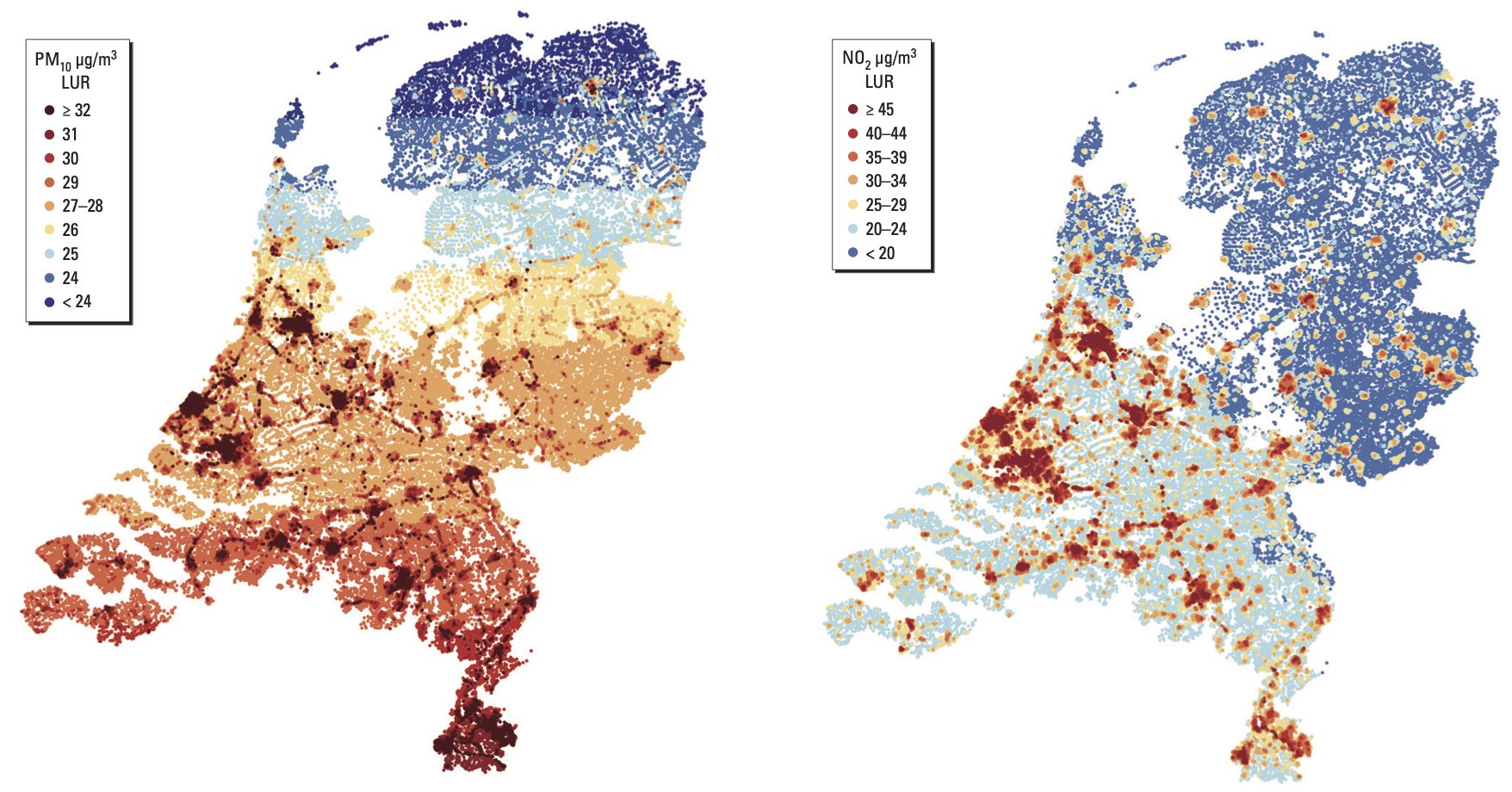
Particle levels and NO2 levels are correlated but imperfectly, which makes it possible to estimate the effects of each separately.
Then, they did a bunch of regressions.
- First, they tried to predict the odds of dying given NO2 levels, controlling for age and sex. In this model, a 10 μg/m2 increase in NO2 was associated with a 6% increase in overall mortality (excluding accidents).
- If they also controlled for the region of origin, marital status, and household income, the increase in mortality dropped to 4%.
- If they also controlled for socioeconomic status, the increase in mortality dropped to 3%.
- If they also controlled for PM10, the increase in mortality dropped to 2%. That’s their final estimate.
A recent meta-analysis (Huangfu and Atkinson 2020) tried to combine the results of many studies like this.
The final estimate was 2%. That’s nice, except:
- How much do we care about a 2% increase in all-cause mortality? Is that worth the nightmare of cooking on an electric stove?
- Many of these studies only looked at adults over the age of 30. What about the impacts on young people?
I did the math and the answers are:
- It’s equivalent to a drop of 0.2 years of life expectancy.
- It makes little difference if you account for this or not. (This is again ignoring issues like asthma in children.)
To get to terms with this, I took the data on how likely someone is to die at each age in the US. I then computed life expectancies where I multiply those probabilities by various ratios. These are the results:
In theory, changes in total mortality have a nonlinear effect on life expectancy. This is what’s shown in the markers in this graph. However, the linear fits (the straight lines) are excellent, and it’s not very important if you start applying the risk at the age of 30 or at the age of zero. That’s because it’s rare enough to die before you’re 30 that multiplying the odds by a small number has little impact on life expectancy.
We can summarize this graph as saying that when you multiply total mortality risk by a factor of r, you decrease in life expectancy by around 10 × (r - 1) years.
Thus, we get that a 10 μg/m3 increase in NO2 is associated with an expected loss of
of life expectancyᵈ. For reference, that’s just a bit lower than what I previously estimated for particulate pollution, where a rise of 10 μg/m3 of PM2.5 seemed to cause a drop of 0.3 years on average. (Though, the PM2.5 number is disability-adjusted, which makes it a bit larger.)
How much does a stove increase NO2?
I wasn’t able to find a meta-analysis, so I did my own. I found 10 papers that studied this question and classified them according to if they are just observational (looking at average values in different houses), if they do regressions to try to disentangle causal factors, or if they do a randomized trial. I also looked at if they measure personal exposure or levels at home.
Here the “contribution” is the estimated increase in NO2 from using a gas stove rather than an electric stove. If they did a separate analysis for gas stoves with pilot lights, I give both as (without/with). In some cases (ᵈ) I had to do some slightly gross approximations to extract the contribution number.
| Paper | Contribution | Stats | Exposure | Data |
|---|---|---|---|---|
| Dockery 1981 | 25.7 | Obs | personal | 9 families on Kansas |
| Fischer 1985 | 17 | Reg | personal | 612 houses in Netherlands |
| Clausing 1985 | 23.5 | Obs | personal | 500 junior high students in Massachusetts |
| Marbury 1988 | (31.0 / 64.4) | Reg | home | 144 homes in New Mexico |
| Schindler 1998 | 4.3 | Reg | personal | 560 people in Switzerland |
| Lee 1998 | (10.5 / 17.4) | Reg | home | 581 homes in Boston |
| Levy 1998 ᵈ | 14.2 | Reg | personal | 533 people around the world |
| Rotko 2001 ᵈ | 5.2 | Reg | personal | 9 people with gas stoves and 167 without in Helsinki |
| Gillespie-Bennett 2008 | 4.2 | Reg | home | 350 homes in New Zealand |
| Paulin 2014 | (7.25 / 12.0) | Trial | home | 39 homes in Baltimore |
Details on each of the studies.
- Dockery: I’m averaging personal exposure of the Husband+Wife+Child (as they put it in the paper). In the homes with gas stoves this is 40.766. In the homes with electric stoves this is 15.06.
- Fisher: They do a regression on personal exposure and get a coefficient of 17. They have other subgroups (specific water heater types) with coefficients of 15, 17, and 27.
- Clausing: They actually did a regression but don’t share the coefficients. I used the observational mean of (46+49)/2 - (22+26)/2.
- Marbury: found 16.21 ppb = 30.96μg/m3 without pilot light and 33.71 ppb = 64.39 μg/m3 with pilot light in activity room. When they just looked at observational stuff, the average in gas homes was 36.15 ppb while in electric homes it was 7.0 ppb. That’s a difference of 29.15 ppb = 55.67 μg/m3. That’s similar enough to the regression to seem credible. There’s no obvious reason this study should have such larger magnitudes than the others. They did notice that people with electric stoves tend to be richer, but they try to account for this and other appliances in the regression.
- Schindler: This wasn’t the point of their study, just a random aside: “To estimate contributions from indoor sources (mainly gas stoves), we first regressed personal N02 concentrations against indicator variables for zones of residence and gas cooking. We then subtracted the estimated contribution of gas cooking (that is, 4.3 μg/m3) from the personal NO2 values of those subjects who had reported that their kitchen was equipped with a gas stove.”
- Lee: They also have observational data where there’s a difference of 14.9 without a pilot light and 23.5 with a pilot light.
- Levy: Observational difference was 27.313 μg/m3 They did a log-domain regression, got a coefficient of 0.32 for gas and 0.39 for the log-outdoor level. The overall mean outdoor level was 29.25 ppb and the intercept in the loglinear model was 1.66. So we can roughly approximate it as NO2 = exp(1.66 + 0.39 ln (29.25) + 0.32 g) where g is a binary variable indicating the presence of a gas stove. The difference of this when g is 0 and 1 is 7.42ppb or 14.17 μg/3 This is a rough estimate.
- Rotko: They also did a regression of in the log domain and got a coefficient of 0.58 for a gas stove and an intercept of 1.899. Suggests a difference of something on the order of exp(1.899+0.580)-exp(1.899)=5.2. This is a very rough conversion and shouldn’t be trusted too much. Also, they only had 9 people who actually used gas stoves. They also have a difference of observational means which is 8.0.
- Gillespie-Bennett: Did estimates in terms of geometric mean ratios. Found that homes with gas stoves contributed a ratio of 1.42 more NO2 in the living room a regression. The overall mean was around 10 μg/m3. So I think that suggests an increase of around 4.2. Again, this is a rough estimate.
- Paulin: 100 homes randomized, 17 got an electric stove, 15 got a hood, 46 got purifiers, 22 left as controls. They found that changing the stove reduced things by 10.0 in the kitchen and by 7.1 in the bedroom. I’m using the average value of 8.55. They found that hoods did nothing and purifiers at most caused a small decrease. Their supplemental information breaks things down and shows that for homes with a pilot light, the mean decrease at 3 months was 12.0 and for homes without a pilot light it was 7.25.
There are various ways you might combine these numbers:
- Mean over all studies: 16.5 μg/m3
- Median over all: 14.1 μg/m3
- Median with pilot light only: 16.3 μg/m3
- Median over without pilot light only: 10.5 μg/m3
I appreciate what these studies are trying to do but they still make me nervous. Whenever they compared people with gas stoves to people with electric stoves, the two populations were profoundly different: They have different incomes, live in different places, and they tend to have different appliances. The regression analyses try to control for that stuff but you can never be sure that you’ve caught everything.
In theory of course, the dream would be to do a randomized trial: Take a group of people with gas stoves, then pick a random subset and go to their house and replace the stove with an electric stove. Then we’d really know that was the causal factor, but obviously it’s impractical.
Except heroes are real: Paulin et al. (2014) did it. They took 100 homes in Baltimore with gas stoves, left 22 as controls, replaced the stoves with electric in 17, installed range hoods in 15, and installed air purifiers in 46. Then they came back 1 week and 3 months later to measure NO2 levels.
This is awesome and the US Department of Housing and Urban Development is awesome for paying $750,000 to do this and if I ever said anything rude about them before, I’m sorryᵈ.
So, I will use a meta-analysis technique of own invention: Use the one randomized trial and throw everything else away. So, we’ll use the estimate a gas stove without a pilot light increases NO2 levels by 7.25 μg/m3 and a gas stove with a pilot light increases them by 12.0 μg/m3. It is at least reassuring that the observational studies are in the same ballpark.
Behold the power of multiplication
Finally, we can estimate the cost of having a gas stove instead of an electric stove. A gas stove without a pilot light decreases life expectancy by
Meanwhile, a gas stove with a pilot light decreases life expectancy by
Can an air purifier fix this?
I don’t know, maybe not.
A standard HEPA filter will do nothing about NO2 since it’s a gas. In theory and experimentally, activated carbon filters can and do remove NO2 from the air.
What we really want to know is how this works in practice: In real kitchens, how effective is activated carbon at removing NO2? There seems to be amazingly little data on thisᵈ.
The best we have again comes from Paulin et al. (2015) who added air purifiers with activated carbon filters to 46 homes in Boston. They found it reduced NO2 levels by 20-25% in the kitchen and 8-22% in the bedroom. This is despite the fact that they used an extremely beefy air purifier, the Austin HealthMate, which costs $715. They bought one for the kitchen and one for the bedroom. Furthermore, they did inspections and found that 85% of the purifiers in kitchen were actually being used. This is not encouraging.
This is just one experiment, but it doesn’t make me very optimistic.
Can a range hood fix this?
That depends on how good your range hood is. Singer et al. (2017) did experiments in nine homes where they tried cooking various things with and without using the range hood. In one house that had a huge hood with a high-volume fan, it reduced levels by around 90%. Two other homes had reductions of 70% and 50%. In the other six, ranged between 10% and 30%.
Why might this number be wrong?
Will you live 53 days longer if you replace your gas stove with an electric one? Probably not, for three reasons.
First, it’s just an average. There’s lots of variability. Second, even the average is probably different for different people. It depends on how much you cook, how old or well-maintained your stove is, how well ventilated your kitchen is, and if you have any respiratory conditions. Third, my estimate of the average average might be wrong. There’s three things that make me nervous:
-
Nonlinearity. Experiments suggest that large doses of NO2 might be much more harmful than small doses. The epidemiological papers tried to look for this and didn’t find evidence of nonlinearity, but they are looking at outdoor levels that don’t get as high as indoor levels can.
-
Causality. The impact of NO2 on health was estimated from observational data, which is always hard. When we looked at Fisher et al. (2015), they started with an estimate of a 6% increase in mortality for each 10 μg/m3 increase in NO2. Tat slowly decreased to 2% as they controlled for more stuff. How do we know we’re done?
-
Other pollutants. Gas stoves put lots of stuff into the air other than NO2, but I’m haven’t considered them here, for various reasons.
- Carbon monoxide. Just burning natural gas by itself seems to create minimal carbon monoxide. Instead, it seems to come from burning the food, which I’ll ignore as it can be done with any type of stove. At the same time, empirical data do suggest somewhat larger concentrations of carbon monoxide in homes with gas stoves. Causality is tricky here—that might be because some stoves are poorly maintained or because gas stoves produce more heat and make it easier to burn food. Carbon monoxide may be harmless below some thresholdᵈ. (As far as I can tall, carbon monoxide isn’t carcinogenic or genotoxic. The problem is simply that it bonds to hemoglobin where oxygen is supposed to go meaning it denies oxygen to cells. That’s very dangerous in high amounts but there’s probably slack in the system for small amounts since Earth’s atmosphere naturally contains traces of carbon monoxide.)
- Particulate pollution. This is definitely bad for you, but the situation is similar to carbon monoxide. In theory, burning methane should create minimal particles—they should be due to burning food rather than the type of stove. But again, experiments suggest that gas stoves do lead to somewhat more particles. For simplicity, I’m going to assume that you already have ventilation or an air purifier in the kitchen so this isn’t a problem. (If you don’t, what are you waiting for?)
- Ozone. This is a problem and gas stoves produce more of it than electric stoves. The issue is that if we try to account for the harms of ozone, that may lead to double counting because ozone (O3) comes from NO2 reacting with other chemicals in the air. That is, when we measure how much NO2 hurts you, ozone may be one of the central mechanisms. So again I’m going to ignore ozone and hope for the best, though note that estimates suggest the dose-response relationship of ozone on mortality is similar to NO2, so this hopefully isn’t screwing things up by more than a small factorᵈ.
Takeaways
If you have young children, please note that I haven’t examined the data there. Lin et al. (2013) do a meta-analysis that suggests it raises the odds of asthma and wheeze.
I think the final estimate of 53 days is probably correct within an order of magnitude i.e. the average is somewhere between 0 days and a year or so. On top of that, there’s lots of person-to-person variability, depending on your stove, your stove usage, your health, etc.
What can you do? Sadly, the things that are guaranteed to work are all fairly hard: Cook less, ventilate better, or get a different stove. Ventilation is great if you have clean and temperate air outside, otherwise highly problematic. Getting a different stove is expensive and infeasible for people who rent.
While it’s not guaranteed to solve the problem, you should be using your range hood anyway for particle pollution. Similarly, you might as well us an activated carbon air purifier, just in case that helps.
The closest thing to a silver bullet is probably to buy portable induction stove, which you can do for as little as $50-$100. Nobody likes old-style “glowing spiral” electric stoves better than gas, but many do prefer induction. That’s probably where we’re all headed eventually.