The Internet is well into middle-age, and yet doesn’t seem to have answered humanity’s most pressing question: If you pour boiling hot water from various heights, how much does it cool in flight?
First attempt
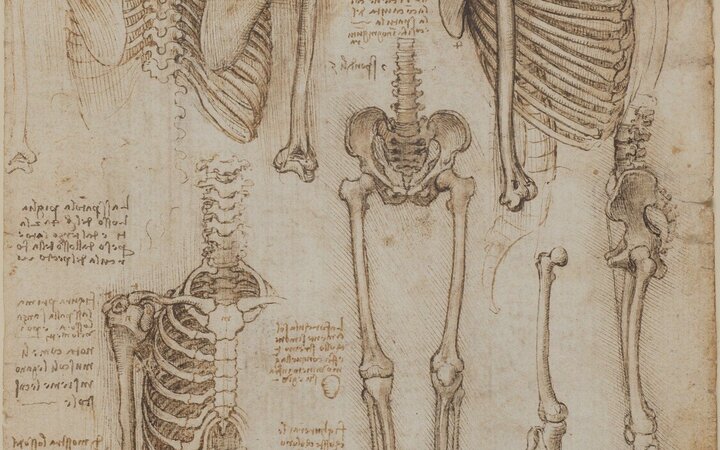
I figure there are two key variables: The height you pour from, and how fast you pour. To control for both of these, I built this little apparatus:
To get different heights, I cut off the top off a 2-liter bottle and drilled two holes in it. I then shoved the end of a clamp through the holes and clamped the clamp to four positions on a plank of wood.
To get different pour speeds, I took three bottle caps, and drilled different-sized holes in them.
Finally, for each height/cap-width combination, I poured some boiling water into the funnel and let it fall into a silicone ice-cube tray. I measured the peak temperature with a digital thermometer, being careful to allow the scalding-hot water to splatter onto my unprotected hand.
First results
I took five measurements in each setting. Here it is with a bit of horizontal jitter when needed to avoid overlap.
This data is quite noisy. My guess is that’s because there’s some surface tension that helps water cling to the bottom of the cap. This makes the stream of water jiggle around chaotically. (This looked the most random at higher heights, though that doesn’t appear to show up in the measured temperatures.)
Here’s a quadratic fit for each of the three cap sizes:
While the offsets vary, the shapes of these curves are pretty similar. So we can summarize these experiments like this:
- Pouring from even a very low height cools water by 3 to 13 ºC, depending on the pour speed.
- Pouring from 25 cm (10 in) gives an additional 1-2 ºC.
- Pouring from 60 cm (24 in) gives an additional 5 ºC.
Also: If you conscript the people you live with into helping you with this experiment, you may find that proposing to repeat it at 100 cm results in shrieks of dismay (“please don’t pour another 10 liters of boiling water onto our counter” etc.) which are not conducive for Important Science.
Second Attempt
But how does this correspond to the real world? I decided to do a second experiment pouring water out of a kettle by hand. This time I used the clamp to hold the thermometer:
I tried to pour at different speeds, but it was hard to be consistent. So I just measured how long it took to fill the (250 ml) cup. Here’s the data:
And here’s a linear fit for each of the three heights:
(I don’t know why there’s no confidence interval for the 8 cm data.)
Finally, I fit a regression. The final fit was
(temp) = 98.0 - 0.21 × (height) - 0.37 × (time),
where (temp) is in ºC, (height) is in cm, and (time) is the number of seconds needed to pour 1 cup (250 ml).
For example, say you pour a liter of boiling water from a height of 10 cm at a rate where it takes you 20 seconds to pour the full liter. That’s equivalent to 5 seconds per 250 ml, so this formula predicts the final temperature will be around
98.0 - 0.21 × 10 - 0.37 × 5 ≈ 94 ºC.
Conclusion
Pouring from higher heights can make water significantly cooler. But to get much cooling from extra height, you need quite a lot of height—so much that you’d have to be very coordinated (or crazy). So for most people, your best bet is probably to pour slowly, to pour into a thick mug so the thermal mass can neutralize more of the heat, or (horror) to wait.
Notes
-
It turns out that 1 plastic ♲ does not melt when exposed to boiling water, though it does become very soft.
-
To cool the funnel between measurements, I poured some room temperature liquid through it. To the horror of the Dynomight Biologist, the closest liquid on hand happened to be a pot of expensive insect-bitten tea that I’d brewed a day earlier and not consumed. It’s hard to be sure what the insects would think about this.
-
Thank you for reading my middle-school science fair project.